

KRYPTON MASS NUMBER FREE
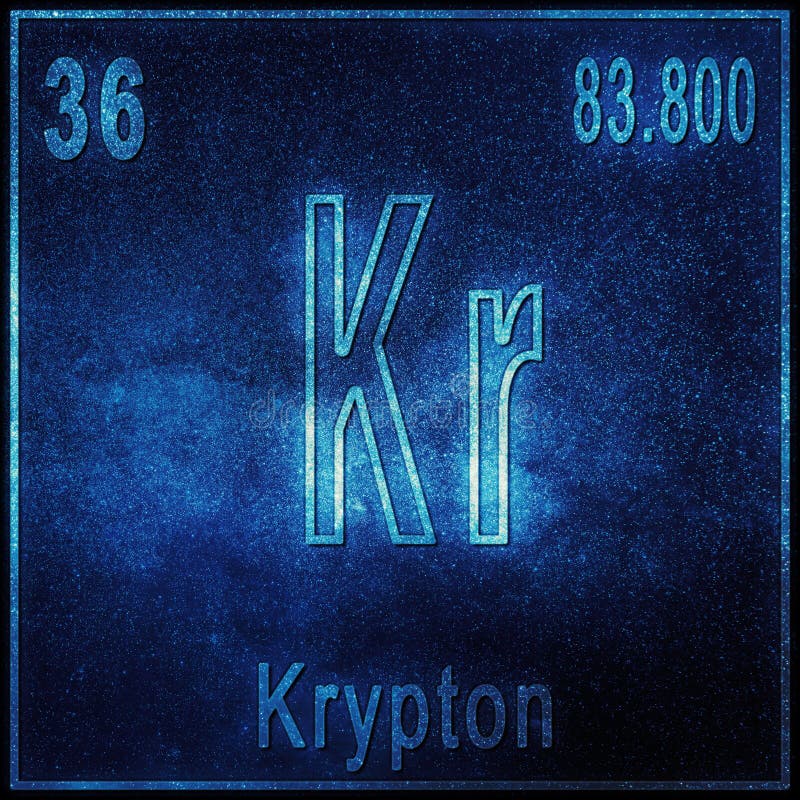
Krypton difluoride (KrF 2) Interesting facts: It is found free in nature. From the Periodic Table we can find the element. In nuclear science, the mass excess of a substance is the difference between the actual mass. Atomic Number: 36: Atomic Mass: 83.8 atomic mass units Number of Protons: 36. In this video we’ll use the Periodic table and a few simple rules to find the protons, electrons, and neutrons for the element Krypton (Kr). We will let 6Li = x and 7 Li = 1-x we use 1 – x instead of 100 – x because the small number is easier to work with. The Isotope mass of Krypton-85 is 84.9125273(21) u. Thermal conductivity / W m-1K-1: 0.0095 IONIZATION ENERGIES. Since I don’t know what the percentage are, I will have to use variables.ġ00% of Lithium is determined by these two naturally occurring isotopes. Density / g dm-3: 2823 THERMAL PROPERTIES. Determine the percent abundance of each isotope.Īw = + + Ħ.94 = + Since the element has an atomic number equal to 36, it follows that it will contain 36 protons in its nucleus. What is the color of Krypton Krypton is Colorless. What is the atomic number of Krypton The atomic number of Krypton is 36.

It is located in group 18 and period 4 in the modern periodic table. Krypton is the 36 element on the periodic table. A 84 This means that the nucleus of a krypton-84 isotope contains a total of 84 protons and neutrons. Krypton is a chemical element with the symbol Kr and atomic number 36.
The atomic mass of lithium is 6.94, the naturally occurring isotopes are 6Li = 6.015121 amu, and 7Li = 7.016003 amu. In your case, krypton-84 is said to have a mass number equal to. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. Protons and Neutrons in Barium Barium is a chemical element with atomic number 56 which means there are 56 protons in its nucleus. Atomic mass for Cu = 63.546Ħ3.546 = + Ħ5Cu = 1 – x = 1 – 0.6916 = 0.3084 x 100% = 30.84% 1 grams Krypton to mol 0.01193 mol 10 grams Krypton to mol 0.11933 mol 20 grams Krypton to mol 0.23867 mol 30 grams Krypton to mol 0.358 mol 40 grams Krypton to mol 0.47734 mol 50 grams Krypton to mol 0.59667 mol 100 grams Krypton to mol 1.19335 mol 200 grams Krypton to mol 2. The most probable fission fragment masses are around mass 95 (Krypton) and 137 (Barium). What are the percent abundances of the isotopes? Since the overall atomic weight for copper is not given in the problem, you must look it up in the periodic table to work this solution. If you look in the periodic table you will be able to check that our answer is correct!ģVerify that the atomic mass of magnesium is 24.31, given the followingĪtomic mass= + + ĭetermining the percent abundance of each isotope from atomic mass.Ĭopper exists as two isotopes: 63Cu (62.9298 amu) and 65Cu (64.9278 amu). 10.81amu so, the atomic weight of B = 10.81amu


 0 kommentar(er)
0 kommentar(er)
